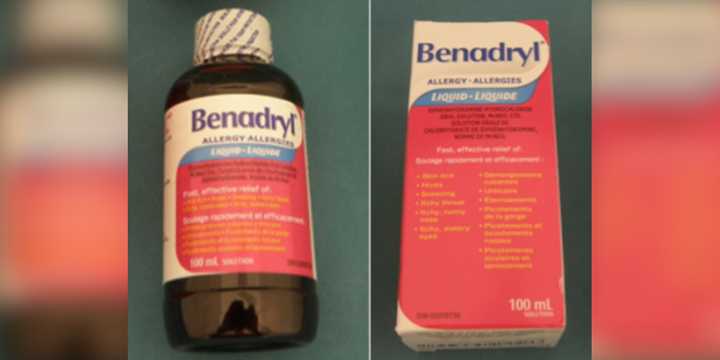
Arsell Inc. is recalling about 2,300 bottles of Benadryl Liquid Elixir, the Consumer Product Safety Commission said in a news release on Thursday, Mar. 20. The Brooklyn, New York-based company issued the recall after discovering the 100-milliliter bottles' packaging wasn't child-resistant, violating federal safety regulations.
The liquid allergy medication contains diphenhydramine, which must be packaged in a way that prevents children from accidentally ingesting it. The affected bottles were sold online at Amazon.com between July 2023 and October 2024 for $16 to $19.
Each bottle comes in a dark plastic container with a pink and white label featuring the word "Benadryl" in blue text. The packaging also includes a paper box with the same branding and a white label on the bottom marked with the code "X003VRIGUL."
Consumers are urged to immediately store the medication out of reach of children and contact Arsell for a full refund. Refunds will be issued after emailing an Amazon order number and a photo showing the product's disposal to recall@arsellsupport.com.
Although no injuries or poisoning cases have been reported, both the bottle and the medicine should be discarded. Arsell is directly notifying all known purchasers.
The recalled bottles were manufactured in Canada.
Click here to follow Daily Voice Sinking Spring and receive free news updates.